Sailing in rough sea
Managing clinical trials is even more like sailing in rough sea. QCTMS PM was designed to fit the needs of project managers. QCTMS PM lets you report relevant information, e.g. financial status and project milestones, with smart forms that can be used even while working offline. QCTMS PM also acquires data automatically from other QCTMS modules, e.g. to track
- randomization status
- eCRF completion
- query resolution
- monitoring report completion
Tailored Analysis
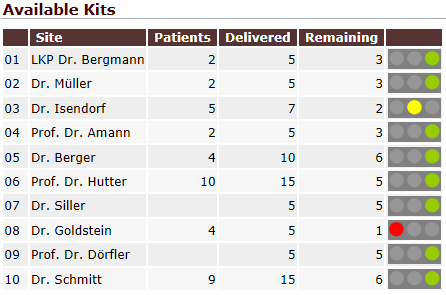
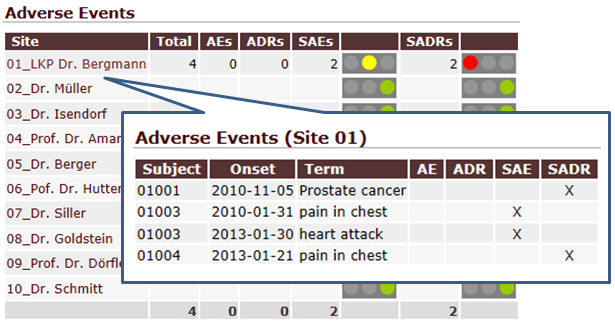
QCTMS PM gathers data and analyzes it to let to gain insights in a more efficient way. You do not need to copy data manually from several spreadsheets. Instead, you are able to access a set of tools that summarize data in tailored way. Trending arrows, traffic lights and drill-down assist you in using even complex tables. Results can be exported as PDF, image file or CSV data for further processing (e.g. Microsoft Excel©).