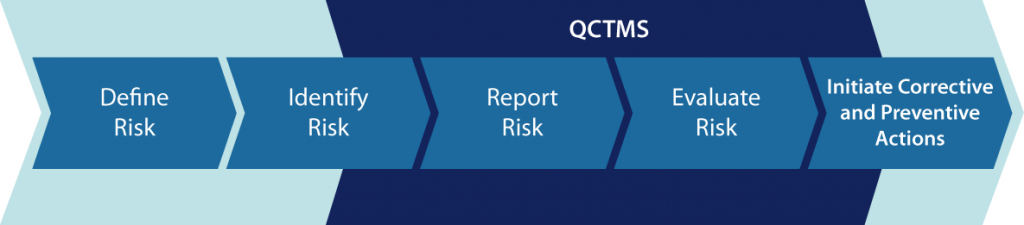
Quality Risk Management (QRM) according to ICH Q9
Effective monitoring and ensuring sponsor oversight are vital for clinical research. But as the complexity and number of clinical trials is rapidly growing, these tasks are challenging more than ever. To ensure patient welfare as well as data quality and integriy both FDA and EMA expect sponsors to implement systematic and risk-based approaches. They recommend approaches according to ICH Q9. QCTMS RM is your tool for QRM according to ICH Q9.
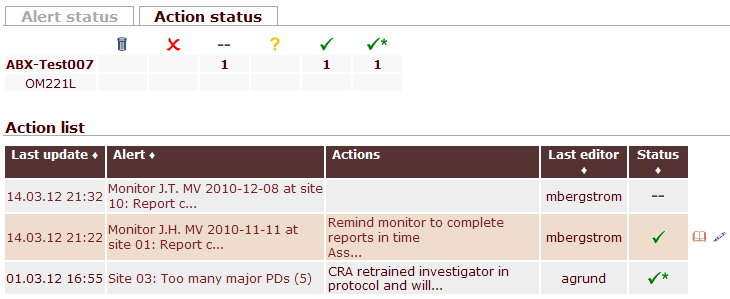
Alerts and CAPAs at a glance
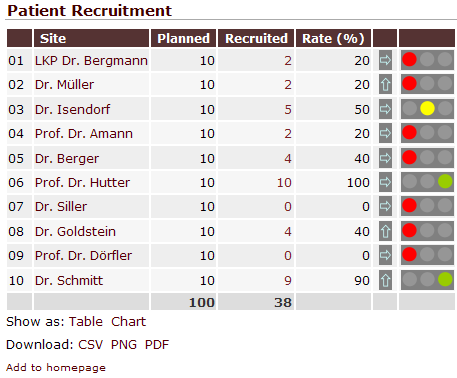
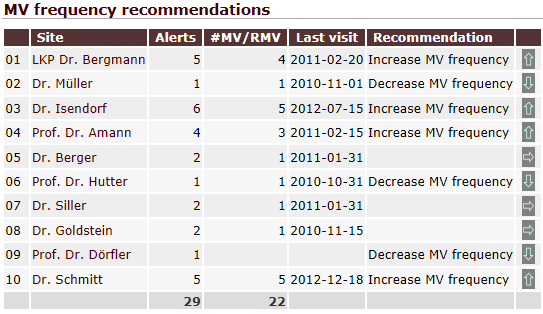
QCTMS RM evaluates risk factors automatically and creates alerts if significant deviations occur, e.g. low recruitment or too many protocol deviations. Additionally, they are automatically sent to all responsible team members. Alerts and the corresponding CAPAs are summarized in an intuitive dashboard.
Tailored risk management
Risk management with QCTMS RM will be tailored to your requirements. This includes the way indicators are calculated as well as the corresponding alerting levels. Track alerts easily and add CAPAs with a slim form that focusses on what really needs to be done. Thus, QCTMS RM lets you efficiently and effectively manage risks even in studies with more than 100 sites or in sites with unconventional requirements.
Effective Monitoring
By evaluating site-specific risk factors you are able to identify sites that require more intensive monitoring. This risk-based approach enables you to decrease monitoring budgets for sites that have proven to work on a high quality level.
Utilizing data instead of reentering data
We are convinced that modern risk management tools should utilize data instead of requiring users to reenter data. This is the reason why QCTMS RM automatically gathers data from all those monitoring reports, electronic CRFs, randomization logs etc.
QRM for SMEs?
Of course! Small and medium sized companies do not have large departments and budgets to complex processes for risk management. Instead, they need tools that help them focus on what is really needed. This is what QCTMS RM has been built for. Our SME customers tell us that we have implemented straightforward processes and that now additional manpower is needed. Our sales would be glad to show you that the budgets for QCTMS RM are also SME-friendly.