Superseding paper CRFs
QCTMS EDC allows your investigators to enter study data with a web-based electronic CRF. For you, this eCRF offers many advantages over paper CRFs:
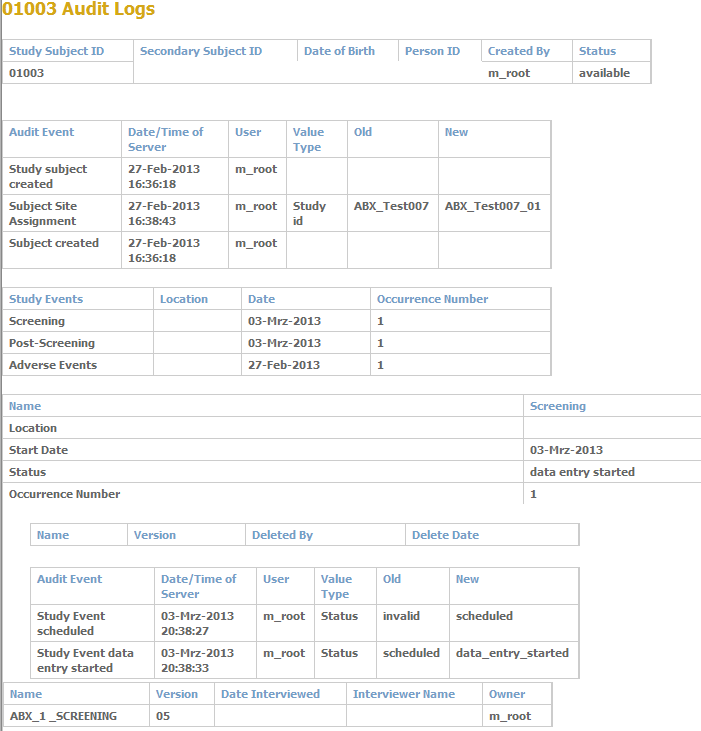
- Built-in checks increase data quality.
- Remote monitoring decreases monitoring expenses.
- Fast data acquisition ensures transparency and improved project management.
Setting up an eCRF with QCTMS EDC is usually less expensive than printing and shipping paper CRFs.
Versatile eCRFs
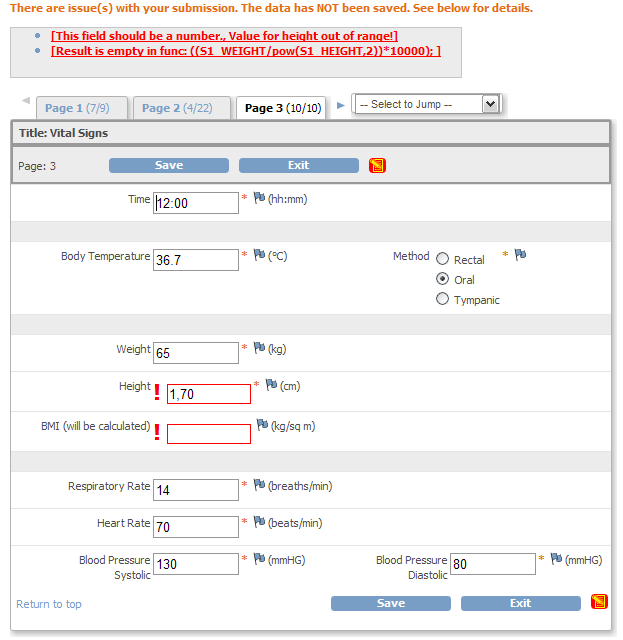
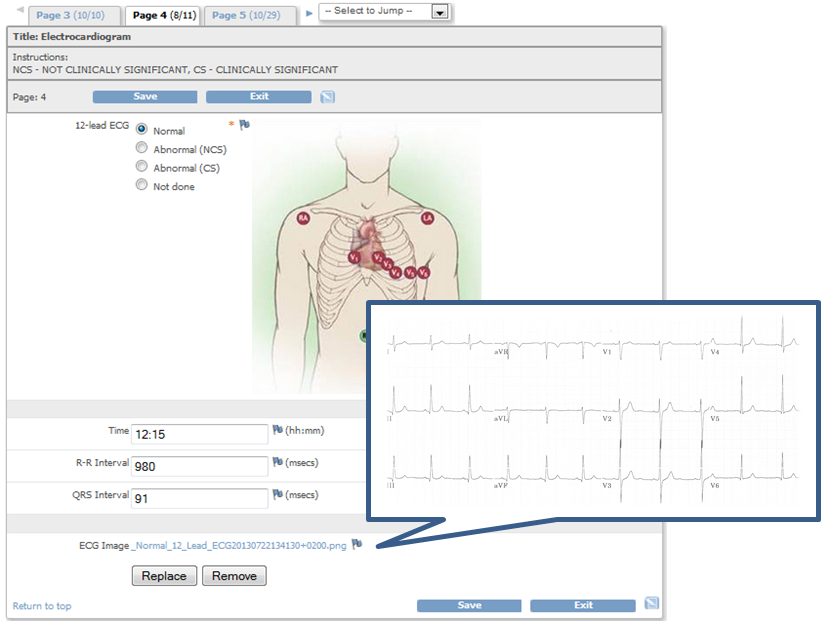
eCRFs built with QCTMS EDC offer all common types of input fields: Text, numbers, dates, checkboxes, lists etc. Additionally, image and document uploads are possible. Complex CRFs can be simplified by dynamically hiding fields that are not needed for all subjects.
Further benefits within the QCTMS Family
As a member of the QCTMS family, QCTMS EDC offers further benefits when using it with QCTMS PM, QCTMS RM, or QCTMS IRT:
- Up-to-date custom reports according to your requirements
- Centralized randomization and efficient kit shipment
- Risk management according to ICH Q9 with CAPA handling
- Risk-based monitoring
Of course, QCTMS EDC offers technical measures to allow for compliance with the applicable standards, e.g. 21 CFR Part 11.
No investments in Servers
QCTMS EDC is provided as a service. You do not need to invest in servers or further software licenses. See our page on Software as a Service for further details.