PRO built for the patient’s daily routine
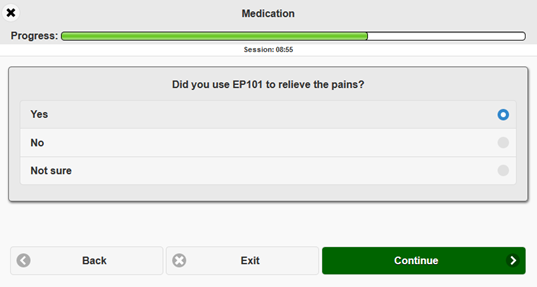
PCQ Pilots has developed QCTMS ePRO as a versatile tool for electronic Patient Reported Outcome (ePRO), which enables you to acquire patient reported trial data through the subjects’ own devices. The subjects can use QCTMS ePRO on common mobile phones, on tablet computers, or on desktop computers.
Increased compliance by using the subjects’ devices
QCTMS ePRO lets the subjects use their own devices. This makes using an ePRO much more convenient for the subjects, because their electronic devices are part of the daily routine. No app installations are needed. Subjects can start using QCTMS ePRO quickly.
Flexible questionnaires
Of course, QCTMS ePRO supports common question types (number fields, text fields, radio buttons, VAS etc.). These can be arranged in blocks and can be shown according to custom rules. Besides that questionnaires can be translated easily. Questionnaires can be assigned to one-time, periodic or occassional events with several reminding options.
Efficient control
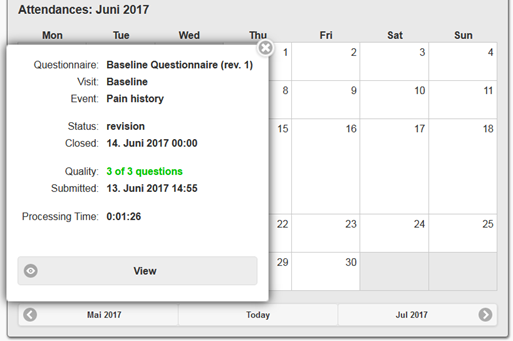
Integrated features for subject compliance and event tracking increase the investigator’s efficiency in using an ePRO system. Being a part of QCTMS compliance data can also be tracked in QCTMS RM.