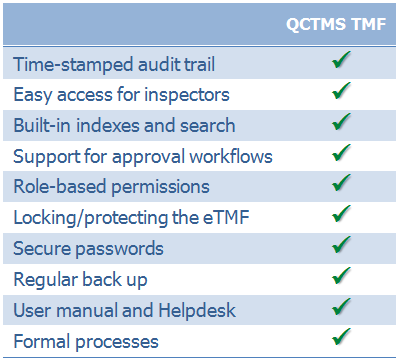
EMA’s reflection paper on GCP compliance related to TMF describes how an electronic Trial Master File (eTMF) should be designed. With QCTMS TMF we offer such a solution.
With QCTMS TMF your study team easily maintains a centralized electronic Trial Master File. Setup the TMF structure according to your SOPs, upload and manage TMF documents in a very efficient way, have an automatically created audit trail, and gain further insights by searching and analyzing your TMF.
Contact our sales team now, and benefit from our special offers for new users of QCTMS TMF.